AMERICA- GSK Consumer Healthcare, a British multinational pharmaceutical company, issued a nationwide recall on Thursday, June 18 to:
- Retail Children’s Robitussin®: Honey Cough and Chest Congestion DM
- Children’s Dimetapp®: Cold and Cough due to the inclusion of incorrect dosing cups.
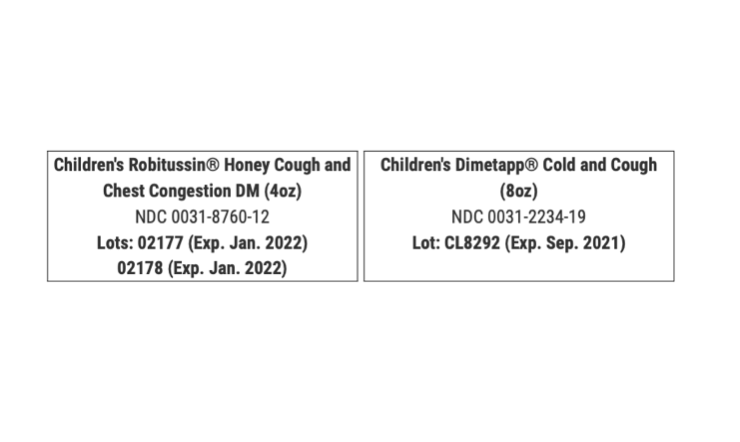
The recall is limited to:
During the review of the packaging documents for these products, GSK discovered that dosing cups for Children’s Honey Robitussin were missing 5 and 10 mL graduations. Graduations classify the concentration of a dosage on medicine.
In select Children’s Dimetapp Cold and Cough products, 10 mL graduation indications were missing on dosing cups. The dosing cups packaged with both Robitussin and Dimetapp products only indicated the 20 mL graduation indication marks. This recall is being conducted with the the U.S. Food and Drug Administration.

GSK declared that there is potential risk of accidental overdose if caregivers dispensing the syrup do not notice the discrepancies between the graduations printed on the dosing cups and the indicated amounts to be administered.
Children’s Robitussin: Honey Cough & Chest Congestion DM contains:
- 10 mg dextromethorphan HBr USP
- Guaifenesin USP 100 mg per 10 mL
- Is labeled for children 4 and older, as well as adults.
Children’s Dimetapp Cold & Cough contains:
- 2 mg brompheniramine maleate USP
- 10 mg dextromethorphan HBr USP
- 5 mg phenylephrine HCl USP per 10 mL
- Is labeled for children 6 and older, as well as adults.
Symptoms of overdose of either product may include elevation in blood pressure, lack of energy and enthusiasm, severe dizziness or drowsiness, fainting; psychotic behavior, or seizures. As of the date of the recall announcement, GSK Consumer Healthcare has not received any adverse events related to these products or consumer complaints regarding the incorrect dosing cups supplied with the product.
These lots were distributed nationwide between February 5, 2020 and June 3, 2020 within the United States. GSK Consumer Healthcare has notified wholesalers, distributors and retailers to arrange for return of any recalled product. Wholesalers, distributors and retailers with an existing inventory of the lots being recalled should stop distribution and quarantine these lots immediately.
Consumers with questions regarding this recall, or with reports of an adverse experience, please call 1-800-762-4675 Monday thru Friday from 8:00am to 6:00pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.